Teaming for Interdisciplinary Research Pre-Seed Program
Why do vaccines against malaria fail in the field?
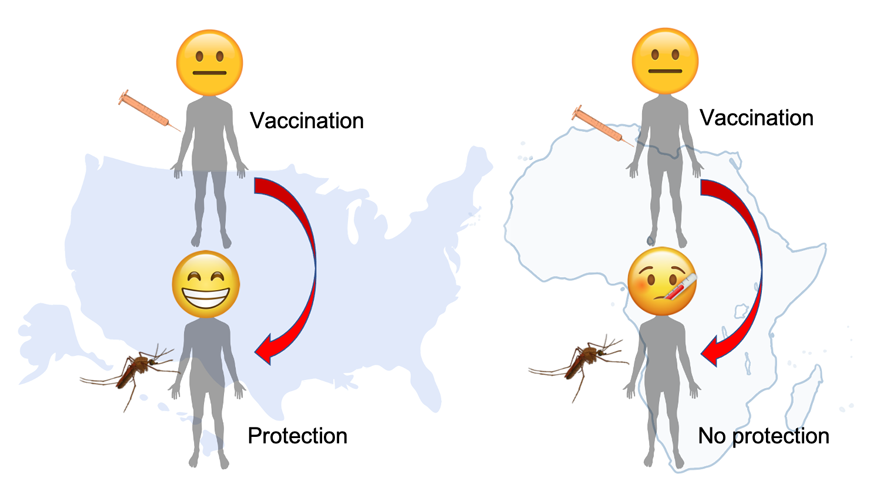
Why do vaccines against malaria fail in the field?

Historically, development of malaria vaccines has typically involved an initial clinical trial in a malarianaïve population, followed by assessments in populations in malaria-endemic areas, such as in Africa. It has been recognized that most malaria vaccines under such clinical trials are typically more immunogenic and efficacious in malaria-naïve populations in the western hemisphere than in malaria-exposed individuals in the tropics. The underlying immune mechanisms that contribute to these differential vaccine efficacies are not fully understood, and is a major impediment to the deployment of vaccines against malaria in malaria endemic regions. While the nature of this “immune hypo-responsiveness” is unknown and may be complex, one key difference is that, the people living in malaria-endemic regions had experienced one or more episodes of clinical malaria infection prior to the immunization trial. We believe that the host immune responses are fundamentally altered due to previous exposure to malaria infection itself and is responsible for the immune hypo-responsiveness to vaccine.
Our hypothesis is that the long-term pathological and immunological alterations in the host as a consequence of malaria infection in the blood would result in dysregulation of natural cell-death pathways in liver cells, that house the live-attenuated anti-malarial vaccine. This would in turn result in a defect in the delivery of anti-malarial vaccine antigens to professional antigen presenting cells. Resolving the mechanisms underlying this process using the mouse and non-human primate models, as well as longitudinal retrospective human data will help design immunological and therapeutic interventions to overcome it, and identify predictive biomarkers of immune hypo-responsiveness.
The Kurup lab has expertise in modeling malaria infection in mice, which would help us dissect the core immune mechanisms behind the phenomenon. The Joyner lab has expertise in investigating malaria using the non-human primate model, which will help interrogate the findings from mice in the context of primate infections, offering significant translational relevance. Drs. Etheridge and Huet are exceptional parasite cellular biologists, who will offer new insights into the role parasite biology itself may play in vaccine failures. Dr. Muralidharan is a Plasmodium molecular biologist and biochemist has who has established protocols and models to undertake targeted gene deletions in Plasmodium. Dr. Peterson uses bioinformatics to predict the biology of malaria, and has collaborative ties with academic groups in Kenya and CDC. The Kurup lab also has ongoing collaborations with the National Institutes of Health, which will provide access to human clinical trial data and materials. We believe that our findings from this collaborative effort will help advance a sustainable vaccine strategy for malaria.
Team Lead
Samarchith Kurup
Department of Cellular Biology
samar@uga.edu
Team Members
Chester Joyner
Department of Infectious Diseases
Vasant Muralidharan
Department of Cellular Biology
Ronald Etheridge
Department of Cellular Biology
Diego Huet
College of Pharmacy
David Peterson
Department of Infectious Diseases
