James was in for a fight.
Battling RSV
Almost all children in the U.S. will be infected by respiratory syncytial virus by age 2, according to the Centers for Disease Control and Prevention. Most of them will recover on their own, but each year about 60,000 kids under age 5 wind up hospitalized with complications like pneumonia or bronchiolitis, an infection of the lungs that causes inflammation, congestion, and narrowing of the airways.
Several hundred children with the most severe complications die, not to mention the thousands of people over 65 who also succumb to the virus every year.
There’s no current treatment for the virus itself. Health care workers can provide supportive therapies—oxygen, intubation, and a machine that breathes for the sick—but the only real healer is time.
For now, there’s also no vaccine to prevent kids from getting sick in the first place. But that’s something the University of Georgia’s Ralph Tripp aims to change.
All RSV vaccines have failed
For more than 60 years, scientists have been working on a safe and effective RSV vaccine. Billions have been spent on development and testing. So far, nothing’s worked.
For many years in the U.S., making a vaccine was a three-step process: Isolate the organism you’re trying fight, kill it, and inject it into the patient. “It mostly worked for many pathogens, but now we’re way past that,” says Tripp, Georgia Research Alliance Eminent Scholar and GRA Chair of Animal Health Vaccine Development. “We’re now taking into account systems biology that includes how the virus replicates, how infections occur that avoid the immune response, and how one can tailor vaccines to achieve the best results.”
The ultimate goal is to make vaccines safer, more effective, and faster and cheaper to mass manufacture.
But first, vaccine candidates have to cross what researchers often refer to as the “valley of death,” the pre-clinical trial stage to determine efficacy.
That’s where all the RSV vaccines have foundered.
Scientists had explored the virus’s genes and pinpointed a protein (known as the fusion, or F, protein) that allows RSV to infect susceptible cells. It seemed like an obvious target. If they could stimulate antibody production against the protein, surely it would stop the disease.
Except it didn’t. In clinical trials, the vaccines would knock out the virus’s ability to replicate within patients, but they still suffered all of the disease symptoms.
“Vaccine manufacturers prefer to take the easiest approach,” Tripp says. “The F protein was well documented and well understood. But the vaccine trials have all failed, and a lot of money has been spent and wasted.”
There has to be a better way, he thought.
“Most of the time the patient does well, similar to many patients with the flu. But there are many, many times when high-risk or even incredibly healthy people get the flu and die. It’s the same with RSV.”
– Carrie Kelly, Pediatrician, Hometown Pediatrics
A very common virus
Every winter, Athens pediatrician Carrie Kelly BS ’00 sees at least one RSV case a day. “Most of the time the patient does well, similar to many patients with the flu,” she says. “But there are many, many times when high-risk or even incredibly healthy people get the flu and die. It’s the same with RSV.”
One of RSV’s most frightening complications is respiratory failure. The baby’s body becomes so tired from the extra work it takes to breathe that it eventually can’t keep up, says Julie Martin, an assistant professor of pediatrics in the Augusta University-University of Georgia Medical Partnership. The infant’s breaths come fewer and farther between. With the drop in blood oxygen levels and buildup of carbon dioxide in the blood, organs begin shutting down.
That’s what was happening with James.
For Anna Claire, the nightmare wouldn’t end. James had been in the hospital for about a week, and he just wasn’t getting better. If anything, he’d taken a turn for the worse. The doctors had to amp up the breathing machine to deliver higher levels of oxygen, but it didn’t appear to be helping much.
Four days after he was first admitted in Athens, James was rushed to Children’s Healthcare of Atlanta, where doctors in the Pediatric Intensive Care Unit began planning their course of action for when his organs began shutting down.
He’d hit what Kelly calls the peak of the RSV cycle, typically about three to six days after the initial illness presents when symptoms are at their worst and most dangerous.
Developing an effective shot
Watching vaccine after vaccine fail in clinical trials was discouraging. But Tripp knew the answer had to be hiding somewhere inside the genetic makeup of the virus.
That’s when it occurred to Tripp and his colleagues that maybe targeting a different protein, the G attachment protein, could be the solution. The Tripp lab previously found a molecule within the G protein that enables it to evade a patient’s immune response, allowing it to infect and replicate throughout the host’s airways. The lab also found that it was essential for the virus to attach to the delicate hair-like cells lining a person’s airways, causing the severe respiratory symptoms.
He had a new vaccine target—and a lot of work to do.
“Vaccine manufacturers prefer to take the easiest approach … But the vaccine trials have all failed, and a lot of money has been spent and wasted.”
– Ralph Tripp, Georgia Research Alliance Eminent Scholar and GRA Chair of Animal Health Vaccine Development
Turning a corner
Back in Atlanta, James had started to turn a corner. He was alert, and his liver had bounced back from near-failure.
A few days later, he was moved from the PICU to the regular floor; a few days after that he got to go home.
Like most RSV cases, as quickly as the severest symptoms of the virus came on, they disappeared.
But for Anna Claire, the emotional scars remain. When she talks about the ordeal, she has to pause and collect herself.
Had a shot existed when James was born, Anna Claire would’ve been the first in line, and any progress in creating one is exciting to her.
“As a mom, you are the biggest advocate for your child,” she says. “If we hadn’t gone to the hospital that day, I don’t know what might’ve happened. I’m just thankful that we did.”
The G protein-targeting vaccine Tripp and his colleagues developed worked well in mouse models and will soon be heading to clinical trials. If all goes well, it could hit the market in a handful of years.
And if Tripp has his way, stories like James’ will soon be a thing of the past.

About the Researcher
Ralph Tripp
Georgia Research Alliance Eminent Scholar and GRA Chair of Animal Health Vaccine Development
Department of Infectious Diseases
College of Veterinary Medicine
Center for Vaccines & Immunology




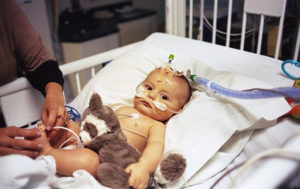 A few days later, James was admitted to Piedmont Athens Regional Hospital, where doctors immediately gave him oxygen, threaded a feeding tube through his nose because he couldn’t nurse and breathe properly at the same time, and inserted an IV through his forehead because his tiny veins couldn’t accommodate the tubing.
A few days later, James was admitted to Piedmont Athens Regional Hospital, where doctors immediately gave him oxygen, threaded a feeding tube through his nose because he couldn’t nurse and breathe properly at the same time, and inserted an IV through his forehead because his tiny veins couldn’t accommodate the tubing.

