In the latter months of 2019, a novel coronavirus probably leaped from a yet-unknown animal in central China into a human.
Some speculate that SARS-CoV-2 leaked from a laboratory in Wuhan, China. But evidence suggests that it’s far more likely that the virus was a natural “zoonotic” leap from animal to human. The resulting COVID-19 pandemic has killed hundreds of thousands of Americans, including more than 23,000 Georgians, and mutated into dangerous new variants.
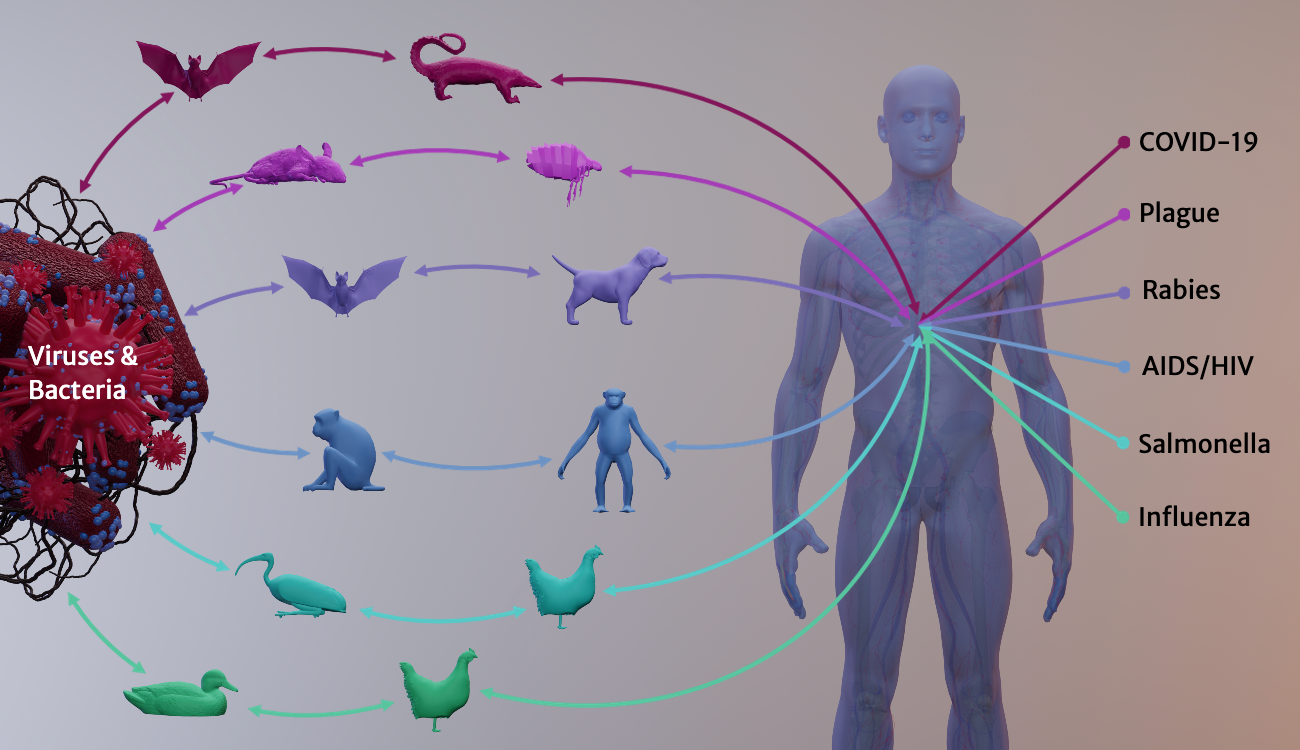
For decades, UGA researchers have been examining the phenomenon of zoonotic spillover—the complex conditions that allow opportunistic animal diseases to infect us. Some spillovers, such as the common cold, cause mild effects. But others can be deadly: plague, rabies, AIDS/HIV and Ebola. Each year, a zoonotic disease—an influenza A virus strain—kills about 1,400 Georgians on average and hospitalizes many more.
“The University of Georgia is one of the leading research institutions in the world tackling zoonotic spillover,” said Justin Bahl, comparative geneticist and associate professor in the College of Public Health and College of Veterinary Medicine at UGA. “We are studying the human and environmental systems in which these zoonotic infections arise and identifying possible solutions.”
Spillover pathogens (viruses, infectious bacteria, parasites and other agents) account for about 75% of emerging infectious diseases that today affect humans. Zoonoses are transmitted when people handle sick animals or their waste, consume diseased animals’ tissues or fluids, or get bitten by infected animals or insects.
Many spillover pathogens originate in animals that live among us as pests, pets or livestock. Cattle gave us measles. Rat fleas hosted the plague, and dogs host rabies. From domesticated swine or poultry, we catch influenza A virus strains, which originated in migratory waterfowl.
A growing number of emerging zoonotic diseases jump into us from wild animals. Viral infections can survive for a long time in wildlife hosts, multiplying slowly and harmlessly. But when humans invade or degrade once-remote habitats, wildlife diseases get the chance to cross the species border to people. Wildlife, livestock operations and human populations increasingly share densely crowded geographies—and share infectious diseases that jump among different species.

UGA scientists are tracking sources and trajectories of zoonotic pathogens to understand where and how they cross species boundaries and cause outbreaks. The goal is to prevent spillover outbreaks before they can happen or turn into epidemics or pandemics.
When people think of infectious disease research, they might imagine lab technicians in protective “space suits” or physicians caring for sick patients in clinical trials—and those types of work are crucial. But other research methods and disciplines are also required to tackle zoonotic disease spillovers.
UGA is well equipped to investigate these types of spillovers because it has broad multidisciplinary expertise and collaboration, according to Bahl.
“We have people like me—a comparative geneticist—studying evolutionary processes of infections. UGA epidemiologists are working to understand infectious disease transmission and virulence in human populations. We have experts who sample the environment to understand which infections are circulating out there. UGA veterinarians and other scientists are working with producers to understand the physiology of domesticated chickens and pigs to reduce opportunities for infections. We have scientists who study the social, economic and public health systems and their contributions to zoonotic spillover,” he said.
“We are studying the evolution, epidemiology and physiology of infectious diseases and interacting human and ecological systems—and we are applying this knowledge to find solutions. The research we are doing can have wide-ranging impacts and long-term consequences for human health. We don’t have to accept the status quo.”
Someday, scientists could learn enough about spillovers to predict new zoonotic outbreaks and forecast their spread. Infectious disease forecasting might become as common as weather reports are today. Imagine timely, localized alerts for COVID-19 or other infections available on a smartphone, identifying hotspots and updating frequently.
“Seventy years ago, weather forecasting was in its infancy, and that’s comparable to where we are right now with infectious disease forecasting,” said John Drake, ecologist and Distinguished Research Professor in the Odum School of Ecology and College of Veterinary Medicine. “We have a long way to go, but the costs of infectious diseases to human lives and the economy are so great that we will make incremental progress toward that goal.”
“Natural systems have equilibriums that maintain the sizes of animal populations. Once humans start changing the dynamics of natural systems, that’s when you can start to have wildlife disease outbreaks and zoonotic diseases and people can get sick.”
– Sonia Hernandez, professor in the Warnell School of Forestry and Natural Resources and the College of Veterinary Medicine
Photo by Amy Ware
Wildlife-human connection
When humans lived in small hunter-gatherer bands, an animal pathogen spilling into people might quickly burn through available hosts and fade away. When humans settled down on farms and villages, we lived with domesticated animals that passed some of their afflictions to us.
As agriculture and commerce intensified, market towns expanded along roadways and waterways. Spillover infections multiplied and spread to other communities. Empire building, wars and trade expanded into new territories, spreading infectious diseases.
Today, logging, farming, mining, and road and settlement construction all bring wild creatures and people into closer contact. Many households in the tropics and subtropics rely on game, or “bush meat,” for animal protein and cash income. Live or slaughtered wild animals are sold in urban “wet markets” where pathogens can move into humans.
“We are changing habitat structures, dynamics and availability of habitat for animals, and those changes come with consequences,” said Sonia Hernandez, professor in the Warnell School of Forestry and Natural Resources and the College of Veterinary Medicine.
Hernandez studies how pathogens affect wildlife populations and ecosystems. “We should not fear wildlife because they may have diseases,” she said. “Instead, we should respect wildlife and be more careful with how we affect their habitat dynamics.”
Some spillovers occur when people eliminate or degrade wildlife habitat while providing abundant food for a reduced population of surviving but stressed animals.
American white ibis, an aquatic waterfowl, has lost wetland habitat across South Florida and the Everglades to urban development, relying more and more on stormwater ponds to roost. The birds congregate in urban parks, competing for food handouts. Human foods disrupt the birds’ gut microbiome and unbalance their immune systems, increasing their vulnerability to intestinal diseases such as Salmonella, UGA researchers found.
Scientists once thought that Salmonella was exclusively a foodborne disease. But UGA researchers have found that humans and wildlife pick up Salmonella from the environment and share it, possibly passing it back and forth. Georgia has the highest rate of human Salmonella cases in the Southeast.
To learn how these zoonotic spillovers occur, Hernandez assembled a multidisciplinary team of UGA researchers, including animal physiologists, disease ecologists, landscape ecologists, microbiologists and disease modelers. The team explores trends in the birds’ habitat use, movement patterns, diet, gut health, immunity and rates of Salmonella infection.
Geography places an important role in understanding this human-bird disease connection. Urbanized white ibis are more likely to carry Salmonella infections than those in natural areas. The research team identified certain Salmonella subtypes in urban ponds where white ibis congregate. Those same subtypes showed up in humans in places where numbers of clinical cases were high.
The birds might catch Salmonella infections indirectly from human activity—that is, from ponds contaminated by stormwater runoff, leaking septic tanks and other sources of untreated human sewage. White ibis might return the infections to humans via the birds’ feces.
“When people feed the birds in parks, the ibis become habituated to that food,” said Hernandez. “The birds climb on benches and picnic tables and defecate, and people might be exposed to Salmonella by touching these surfaces, but we don’t know where humans get these cases of environmental Salmonellosis.”
Wild aquatic birds are similarly attracted to wild bird feeders, spreading Salmonella to backyard chickens and infecting people who handle them.
Conserving habitats could lessen physiological stresses that lead to infection outbreaks in wildlife populations and zoonotic spillovers. “Natural systems have equilibriums that maintain the sizes of animal populations,” said Hernandez. “Once humans start changing the dynamics of natural systems, that’s when you can start to have wildlife disease outbreaks and zoonotic diseases and people can get sick.” Biodiversity, then, establishes healthier conditions for wildlife—and people.
“Wildlife conservation and human health are tightly interlinked,” said Richard Hall, assistant professor in the Odum School of Ecology. “If we can maintain natural foraging and movement behaviors, and preserve and restore natural habitats, we can reduce risk of elevated disease transmission within wildlife populations, with the bonus of reducing spillover risk into people.”

Food, habitats and pathogens
The world’s human population has doubled since the early 1970s, becoming more urbanized and often more prosperous. As the global middle class has expanded, households have additional discretionary income to supplement their diets with meat and meat products.
In Southeast Asia, many households raise backyard poultry in open-air operations. Chickens, ducks and geese are susceptible to influenzas that originate in wild migratory waterfowl, which carry these infections benignly in their guts.
After long migrations, hungry waterfowl flock to sites with abundant food and release virus-laden feces. “When you feed chickens, you can attract large numbers of wild migratory birds to those food resources,” said Hernandez. “You are artificially aggregating wild animals at densities that normally you don’t see in natural systems. And when you have large concentrations of both domestic animals and wild animals that don’t naturally come together, you can facilitate disease transmissions.”
A household operation in Southeast Asia might contain a variety of birds, said Bahl. “They might trap and pen migratory birds, breed wild birds in captivity and also raise classically domesticated animals such as chickens and ducks, allowing them to exchange their genetic materials and spreading highly infectious variants.”
Some viruses, such as influenzas, are so successful partly because of their incredibly rapid evolutionary cycles. “When these viruses get into animals such as domestic birds, they can go through many replication cycles in a short period, burning through that population very quickly,” said Bahl. “They’re among the fastest-evolving viruses that we know, and within that large pool of viruses, you can get new variants.”
Influenza viruses often leap from wild birds to an intermediate host such as domesticated poultry or pig. Eventually, the virus can mutate into a new viral concoction that allows it to leap into a human host and replicate.
But a zoonotic virus can also reverse direction, Bahl noted, spilling back into its previous host. Some influenza variants that jump from wild birds to pigs or poultry spill back into wild birds.
“Variants that do return to wild birds may be different enough from the original avian virus that they can out-compete the ancestral viral population” and become more infectious or virulent, said Bahl.
Aquatic migratory birds can carry evolutionarily super-charged variants across pathways between Asia, Europe and North America where virus variants can spread. European nations and the United States have been expanding pig and poultry farms and meat processing centers into massive operations. Influenza viruses from humans, birds and pigs can mix and mutate into more infectious or virulent variants that can spread back to other species, including us.

How might scientists predict zoonotic outbreaks?
Public health measures and medical interventions have been spectacularly successful in fighting infectious diseases over the past century, lengthening human life spans with improved sanitation, hygiene, treatments and vaccines.
Now, researchers are developing innovative, multidisciplinary research strategies to fight new challenges of infectious diseases.
UGA researchers are integrating social sciences into spillover models to understand how economic incentives can affect the emergence of infections. Poverty can drive people into the wildlife trade or other ventures that allow zoonotic pathogens to emerge.
“We need to study how economic incentives affect decisions to get into a contact zone with wildlife, including why people engage in wildlife trade and wildlife habitat conversions,” said Susana Ferreira, professor in the College of Agricultural and Environmental Sciences. “This kind of interdisciplinary research is important because it better incorporates people’s actions and interactions and socioeconomic drivers into models of spillover risk and disease transmission.”
Ferreira works with a research team led by Patrick Stephens of the UGA Center for the Ecology of Infectious Diseases (CEID), which received a $2.4 million, five-year grant from the National Institute of Allergy and Infectious Diseases to study this very phenomenon. The researchers will investigate and model the origins and impacts of zoonotic spillovers, focusing on Ebola and related filoviruses.
This research is one of many projects by the CEID’s multi-disciplinary Spillover Working Group, which studies drivers and geographies of emerging infectious diseases.
Ferreira’s team will track communities and regions where armed conflict is forcing people from their homes into wild areas to find food and shelter, raising opportunities for zoonotic disease outbreaks.
“Armed conflict can result from economic competition for resources,” said Ferreira. “It can displace people to the forest frontier to look for sources of protein, increasing the consumption of bush meat that could harbor potential zoonotic diseases. Armed conflict can destroy the medical infrastructure that helps us detect and treat zoonotic spillover cases and prevents those cases from spreading into epidemics.”
Ecology is an effective way of thinking about the many complex systems that drive zoonotic spillovers.
“Ecology takes a holistic approach to understand a system,” said Drake, CEID director. “Rift valley fever, Ebola virus and avian influenza have different hosts. But these diseases all result from animal population dynamics, behavior and habitat. Zoonoses result from interactions between people and animals in the environment or on the farm. These interactions have a geographical aspect, spatially distributed in fields, forests and ecosystems.
“Ecology allows us to identify and quantify common risk factors for zoonotic spillovers, so we can make decisions about managing those risks.”
In March, Drake and his colleagues at CEID launched the Global Infectious Disease Intelligence Consortium in March 2021. They want to create partnerships that allow closer collaboration and communication between academic scientists and people in other sectors of the economy who could benefit from the information researchers have about emerging diseases, according to Drake.
“It has been eye-opening to us during the COVID-19 pandemic that relatively large companies lack the kind of information they need about managing epidemics or understanding characteristic patterns of epidemics. We want to work with those organizations to anticipate, identify and rapidly respond to emerging disease threats as we do now with meteorological threats,” he said.
“Although we cannot predict a tornado’s path, we understand the weather conditions under which tornadoes arise. We have built life-saving information tornado warning systems around this knowledge,” Drake said. “Someday we will be able to identify and understand conditions that enable infectious diseases to emerge and make similarly life-saving predictions.”






